Ⅰ Solid-state battery introduction
The solid-state battery is a kind of battery technology. Unlike lithium-ion batteries and lithium-ion polymer batteries that are commonly used today, solid-state batteries are batteries that use solid electrodes and solid electrolytes.
Since the scientific community believes that lithium-ion batteries have reached their limits, solid-state batteries have been regarded as batteries that can inherit the status of lithium-ion batteries in recent years. The solid-state lithium battery technology uses glass compounds made of lithium and sodium as conductive materials, replacing the electrolyte of the previous lithium battery, and greatly improving the energy density of the lithium battery.
In solid-state ionology, a solid-state battery is a battery that uses solid electrodes and solid electrolytes. Solid-state batteries generally have low power density and high energy density. Because of the relatively high power and weight of solid-state batteries, it is an ideal battery for electric vehicles.
In 2020, the research and development of solid-state battery technology are expected to make breakthroughs, and it will further catch up with lithium-ion battery technology in terms of cost, energy density, and production process.
In 2030, lithium-ion batteries will no longer be the mainstream of electric vehicle batteries, but they still have a place in certain electronic components.
Ⅱ Solid-state battery history development
Since Sony brought lithium-ion batteries containing liquid electrolytes into the application of electronic devices in 1991, liquid lithium batteries have become one of the most mature and widely used technical routes.
In 2010, Toyota introduced a solid-state battery with a cruising range of more than 1000KM. The efforts including QuantumScape and Sakti3 are also trying to replace traditional liquid lithium batteries with solid-state batteries.
Canadian Avestor Company also tried to develop solid-state lithium batteries, and finally filed for bankruptcy in 2006. Avestor uses a polymer separator to replace the liquid electrolyte in the battery, but it has not solved the safety problem. There have been several battery burning or explosion incidents in North America.
In mid-March 2015, James Dyson, the inventor of the vacuum cleaner and founder of Dyson in the United Kingdom, invested his first $15 million investment in solid-state battery company Sakti3, which was established in 2007 Battery startup company.
In January 2018, a breakthrough new battery technology seemed to be finally approaching reality. If it meets expectations, the new technology can meet the needs of mobile phone addicts for several days and increase the mileage of electric vehicles to more than 500 miles (about 804 kilometers). This new technology is called solid-state battery technology, and it replaces the liquid electrolyte in today's batteries with ceramic materials.
In January 2018, it formed an alliance with BMW, which has promised to provide some form of battery assembly for every product it produces in the next 10 years, whether it is a traditional hybrid car, a plug-in electric car, or a pure battery. Electric vehicles (BEV).
Ⅲ Working Principle of Solid-state battery
The traditional liquid lithium battery is also vividly called the "rocking chair battery" by scientists. The two ends of the rocking chair are the positive and negative poles of the battery, and the middle is the electrolyte (liquid). Lithium-ion is like an excellent athlete, running back and forth on both ends of the rocking chair. During the movement of lithium-ion from the positive electrode to the negative electrode and then to the positive electrode, the charging and discharging process of the battery is completed.
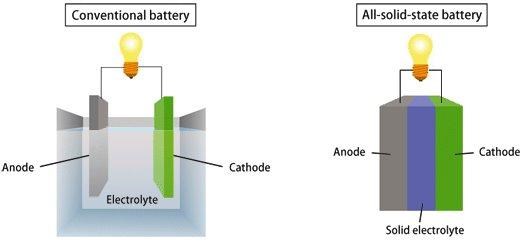
conventional battery and solid-state battery
The principle of a solid-state battery is the same, except that its electrolyte is solid, with a density and structure that allows more charged ions to gather at one end, conduct more current, and thereby increase battery capacity. Therefore, with the same amount of power, the volume of solid-state batteries will become smaller. Not only that, because there is no electrolyte in the solid-state battery, it will be easier to seal it. When used in large equipment such as automobiles, there is no need to add additional cooling tubes, electronic controls, etc., which not only saves costs but also effectively reduces the weight.
Ⅳ Advantage of Solid-state battery
1.Light-high energy density. After the use of all solid electrolytes, the applicable material system of lithium-ion batteries will also change. The core point is that it is not necessary to use lithium-intercalated graphite anodes, but directly use metal lithium as the anode, which can significantly reduce the amount of the negative electrode material and significantly increase the energy density of the entire battery.
2.Thin-small size. In traditional lithium-ion batteries, separators and electrolytes are required, which together account for nearly 40% of the battery's volume and 25% of its mass. And if they are replaced with solid electrolytes (mainly organic and inorganic ceramic materials), the distance between the positive and negative electrodes (traditionally filled with diaphragm electrolyte, now filled with solid electrolyte) can be shortened to even a few micrometers, so that the thickness of the battery can be greatly reduced. So all-solid-state battery technology is the only way to miniaturize and thin-film batteries.
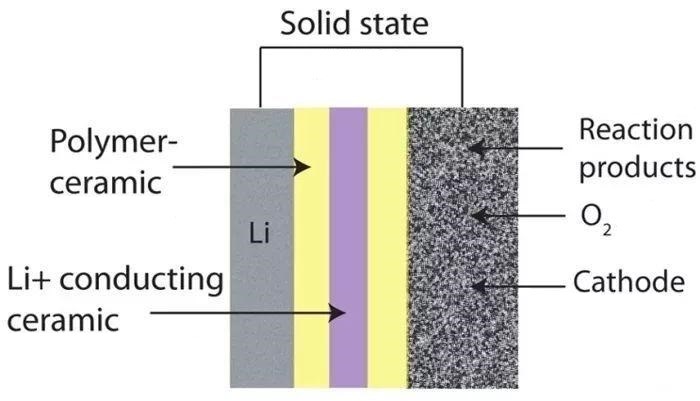
solid-state battery materials
3.The prospect of flexibility. Even brittle ceramic materials can often be bent after the thickness is as thin as millimeters or less, and the material will become flexible. Correspondingly, the flexibility of the all-solid-state battery will be significantly improved after being thinner and lighter. By using appropriate packaging materials (not a rigid shell), the manufactured battery can withstand hundreds to thousands of bends to ensure performance.
4.Safe. Traditional lithium batteries may have the following hazards: (1) Lithium dendrites may appear when working under high current, which will pierce the diaphragm and cause short-circuit damage. (2) The electrolyte is an organic liquid. Side reactions, oxidative decomposition, and combustion will likely occur at high temperatures. Using all-solid-state battery technology, the above two problems can be directly solved.
Ⅴ Controversy of Solid-state battery
Solid-state batteries may be one of the development directions of battery technology in the future, but they may not be the best. "Technical personnel of the aforementioned new energy production enterprises said, "Including fuel cells, supercapacitors, aluminum-air batteries, and magnesium batteries, there is a lot of room for development in concepts, but in the end, it depends on which route develops faster and is more commercially available. The scale and cost of the transformation can reach a perfect balance point. "First, the materials used must not be high-cost and rare. Secondly, it must be possible to achieve large-scale applications in various industries and fields.
Perhaps the most challenging thing now is the price. The cost of liquid lithium batteries is about 200-300 US dollars/kWh. If the existing technology is used to manufacture solid-state batteries that are sufficient to power smartphones, the cost will reach 15,000 US dollars, and the cost of solid-state batteries that are sufficient to power cars can reach even more-a staggering 90 million dollars.
Sastry said that an important reason for the high production costs of solid-state batteries is low production efficiency. According to Sastry's plan, Sakti3 will eventually reduce the cost of the battery to $100/kWh, but she did not give the final time.
From the perspective of the time the theory was put forward, solid-state batteries are not a new concept, but over the years, the progress in research and development has not been as fast as imagined. A technician from South Korea's Samsung believes that even if Sakti3 can eventually achieve cost reductions, it will take a long time for the battery to go from the laboratory to the final mass production. Just like liquid lithium batteries, in the 1970s, related concepts and experimental certifications were advancing side by side, but the real large-scale use was already at the end of the 20th century.
Ⅵ The relationship between solid-state batteries and cobalt
Entering 2021, NIO will focus on solid-state batteries, while Panasonic intends to develop cobalt-free batteries. So, what is the relationship between solid-state batteries and cobalt-free batteries? Are solid-state batteries cobalt-free batteries?
The electrolytes of early solid-state batteries were polymer electrolytes, with PEO (polyethylene oxide) accounting for the vast majority. The electrochemical stability window (oxidation potential) of PEO is 3.8V, which cannot be compared with high-voltage cathode materials (lithium cobalt oxide, Ternary materials, etc.) are compatible, and only lithium iron phosphate can be used as the positive electrode, so the saying that cobalt is not used has been passed down.
However, the early solid-state batteries had a shortcoming, that is, the energy density was not high. For example, the solid-state battery in Bolloré, France, is a PEO electrolyte + lithium iron phosphate cathode, with an energy density of only 170 wh/kg and an operating temperature of up to 80 degrees. Therefore, it has not been promoted, and only 3,000 cars have been installed.
In other words, the early solid-state batteries were actually lithium iron phosphate solid-state batteries, without cobalt, it is not an exaggeration to call it a cobalt-free battery.
With the development of battery technology, most solid-state batteries currently in research and trial production use cobalt-containing cathodes, mainly lithium cobalt oxide and ternary materials. The core of solid-state batteries is the electrolyte, which is currently divided into two categories: polymerization electrolyte and inorganic electrolyte. There are two kinds of inorganics: oxide and sulfide. Their electrochemical windows are roughly 5.4V and 5.7V respectively. Lithium cobaltate and ternary materials can be used to improve the high density and performance of the battery. Although there are other types of cathode materials understudy, they are far from mature with lithium cobalt oxide and ternary materials.
The use of lithium cobalt oxide and ternary materials as the positive electrode can greatly increase the energy density of the battery. For example, Toyota, its cathode material is also lithium cobalt oxide, with an energy density of 400wh/kg;
So here is a conclusion: a solid-state battery with lithium cobalt oxide and ternary materials as the positive electrode contains cobalt, and this solid-state battery is not a cobalt-free battery.
Obviously, the current solid-state batteries containing cobalt are superior to the early solid-state batteries without cobalt. There is a saying that in the foreseeable future, solid-state batteries must use cobalt, and the proportion is even higher than that of lithium-ion batteries!
-
Tel
13824346118 -
Whatsapp

 ENGLISH
ENGLISH 简体中文
简体中文